NDA halts supply of cancer-causing drugs
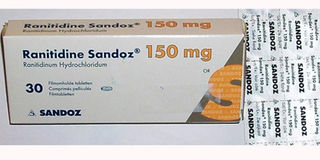
The packaging of Ranitidine, a drug that is used to treat ulcers. NDA has halted its supply following concerns that it may be having Nitrosamine, an impurity that causes cancer. PHOTO | COURTESY
The National Drug Authority (NDA) has halted distribution of drugs suspected to have nitrosamine, an impurity that causes cancer.
The two drugs are Ranitidine, which is used to treat patients with ulcers, and Angiotensin II receptor blockers used in hypertensive patients.
Some pharmacies in Kampala visited by Daily Monitor confirmed they no longer stock the drugs because they are not being distributed.
In a statement sent to Daily Monitor last Friday following inquiry on the actions being taken by NDA to protect the population from the said drugs, the drug regulator said: “Distribution was halted for some of these drugs until manufacturers conduct a risk assessment to evaluate the level of these impurities in their products.”
Those that were already in circulation have, however, not been recalled.
In April, the US Food and Drug Authority asked manufacturers to withdraw all prescription and over-the-counter Ranitidine drugs from the market immediately.
NDA is depending on the manufacturers of the said drugs to take voluntary action of withdrawing the drugs.
“No new imports are being allowed from manufacturers that have not demonstrated the absence of this impurity in their medicines. A number of these medicines have already been authorised in the market after the evaluation of the manufacturers’ corrective action,” NDA said in the statement.
The drug regulator stated that the deadly impurity is still a big problem to them.
“The occurrence of this impurity is not something that could have been excluded by testing at the National Drug Authority’s end, because you don’t test for what you don’t know,” NDA stated.
“Instead as mentioned above, this incident has triggered several reforms in the global regulatory and pharma industry arena to ensure medicines are free from this category of impurities,” it added.
The authority also said it is also adopting a risk-based approach in testing medicines coming into the country because testing is only part of the quality assurance methods to ensure quality.
NDA also said sensitive analytical methods are being developed to ensure reliable detection of impurities such as Nitrosamine.
According to NDA, nitrosamines are molecules of concern that were discovered in certain medicines, occurring as impurities and probable human carcinogens, meaning they have the potential to cause cancers in humans.
“Although they occur in foods, drinking water, and the natural environment, their presence beyond certain limits in medicines is unacceptable because medicines are given to already sick people, hence any harmful substances in these medicines would cause more harm,” NDA added in the statement.
Nitrosamines are a global problem, not experienced only in Uganda. The exact way the impurities get into the drugs is still unclear.
Although some experts think the possibility for nitrosamine impurity content was due to the emergence of substances called nitrites and amines during the manufacturing processes, the World Health Organisation (WHO) thinks it is more.
About the drugs
Ranitidine, sold under the trade name Zantac among others, is a medication that decreases stomach acid production. It is commonly used in treatment of peptic ulcer disease, gastroesophageal reflux disease, and Zollinger–Ellison syndrome.
Angiotensin II receptor blockers are used are in the treatment of hypertension, diabetic nephropathy (kidney damage due to diabetes) and congestive heart failure.