Let’s have conflict-free drug development
What you need to know:
- The issue: Drug development and approval
- Our view: Emergency or not, the process of regulation and approval of drugs and other goods for public consumption should be respected by all concerned.
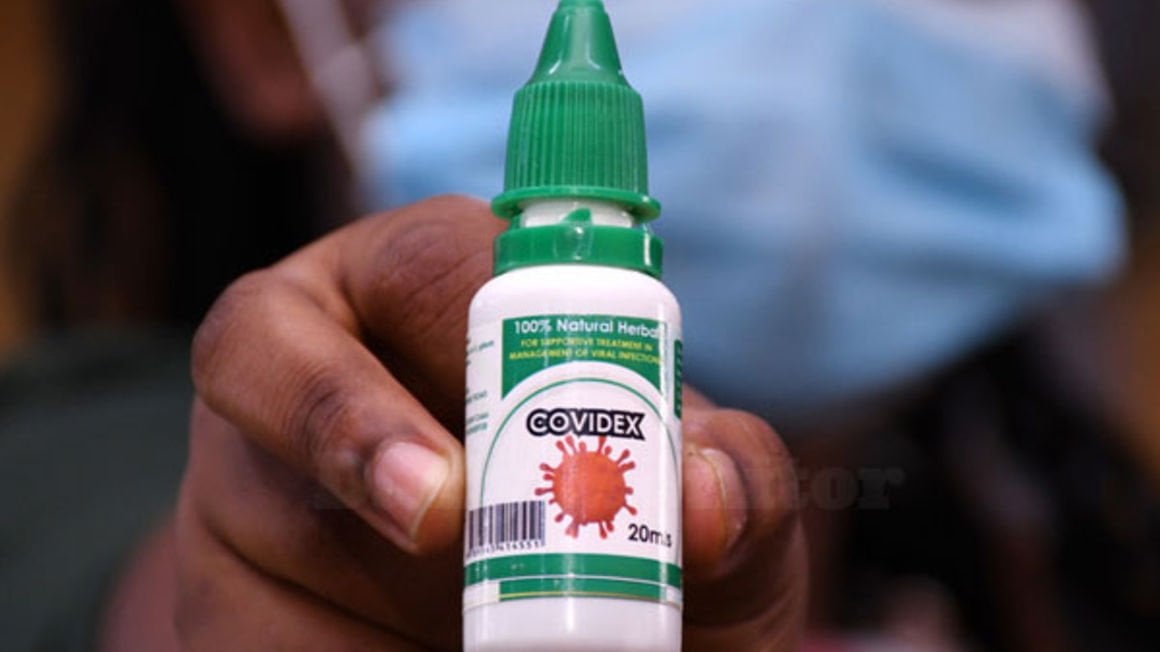
The anxiety, followed by scarcity and now downright rush after Covidex, the drug supposed to support healing in Covid-19 patients tells a story of how we handle drugs in development or don’t, in some cases, until it is too late and the confusion and misinformation has already spread.
The Two Covid drugs, one still not sanctioned by the National Drug Authority, are at the centre of growing controversy on drug development in the country.
As of yesterday’s news reports, National Drug Authority was actively engaged in efforts to stop the team at Gulu University from going ahead to distribute and administer the drug named Covilyce-1. Reports indicated this herbal formula had already been given to at least 100 people who are alleged to have recovered.
On the other hand, a court battle is in the offing over the approval of Covidex and ownership of rights over the drug. The two developments have brought to light the system cracks in regulation of drug development.
Developers seem to be caught on the wrong side of regulation whether as a result of trying to cut through red tape or inadequate knowledge of how the system works. Whatever the case, these very public fights on rights and regulations have left the consuming public at a loss and possibly scaring off any other developers waiting in the wings to unveil other scientific discoveries. This lack of clarity or flouting of the rules, whatever the case may be, needs to be addressed perhaps chiefly through the National Drug Authority’s communication and information flow as well as a resolution of process management flaws.
As it stands, the long awaited scientific breakthroughs have landed in a hotbed of confusion and squabbles over interests, diverting the efforts towards medical advancement as scientists are caught up in administrative roadblocks which could easily be eliminated by creating and sharing information on channels through which innovators can legally bring their products to the market without every invention turning into a fight.
Our higher institutions of learning also need to review their communication systems to ensure that as the noble works of innovation are taking off, the administration is aware of the staff’s activities and the rules are clear on rights ownership. Scientific discoveries can turn out to be very profitable, so managing the commercial interests should not be left for the last minute. Provisions should be in place at the earliest opportunity to provide for that.
Science should not be restricted to the laboratory. The scientists should be in conversation, not just with each other, but with the regulators, through whom their work, if successful, finds its way into the public domain. Emergency or not, the process of regulation and approval of drugs and other goods for public consumption should be respected by all concerned.




