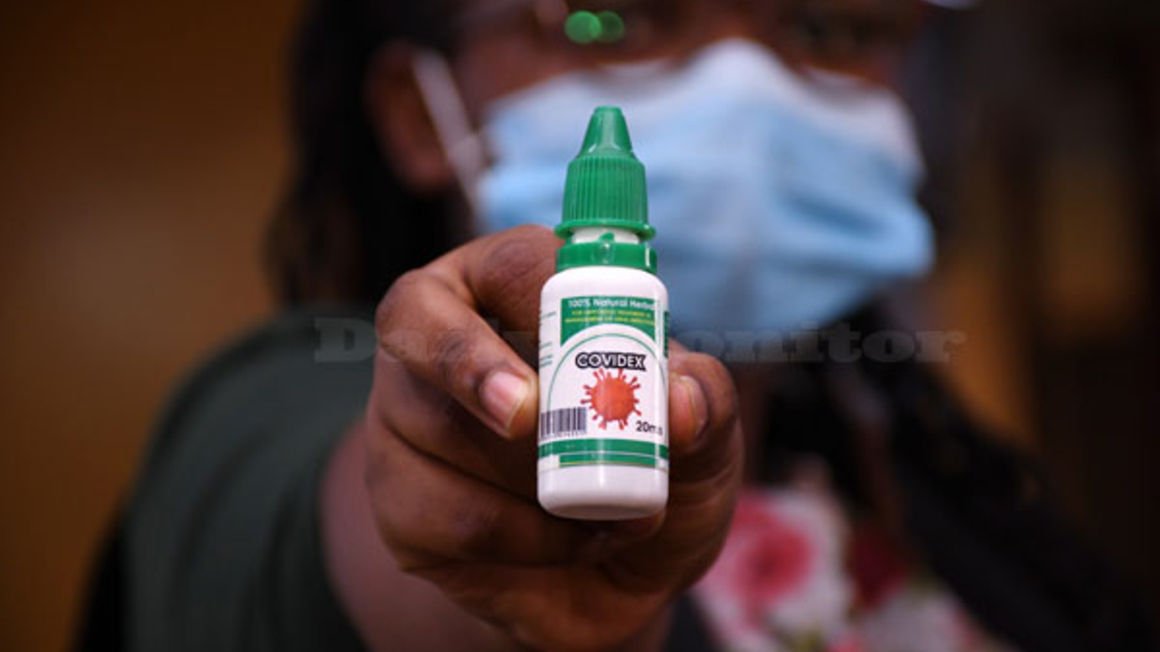
Covidex, Covilyce and NDA; what’s missing?
What you need to know:
- Soon Covidex worked like magic, NDA bowed to pressure and allowed the use of the same in the treatment and management of Covid-19
Since Associate Prof Patrick Ogwang of Jena Herbals Uganda Limited and Mbarara University of Science and Technology brought forth Covidex, the National Drug Authority (NDA) - a body established by law to ensure the availability of quality medicines in Uganda - quickly warned the general public against the unregulated use of Covidex on the assumption that drug was not an approved herbal medicine.
Soon Covidex worked like magic, NDA bowed to pressure and allowed the use of the same in the treatment and management of Covid-19. As a person who has lost two loved ones to Covid-19, I usually encourage anyone to use and do whatever they know so as to boost their immunity against the virus.
Three friends, who battled covid-19 recently, approached me and testified that Covidex worked like a charm but even the best charm does have its own benefits and side effects. As we further descend into the role of NDA in the manufacture, distribution and quality check in quality medicines, let’s not forget the importance of clinical trials.
A clinical trial is the process in which a new medicine is tested overtime to justify its benefits in treating and managing health complications, diseases, infections etc. and this enables researchers to understand the benefits of the medicine vis-à-vis its side effects. Furthermore, clinical trials ensure that medicines availed to the public are the most effective towards the treatment and management of the disease they were established for. This research process usually lasts several years involving many study participants with different backgrounds and characteristics.
What NDA now seeks before herbal medicines are approved and made available to Ugandans is a research process that can show that the herbal medicine has been scientifically tested and it has demonstrated to be the most efficacious and only a clinical trial can answer that question.
But truthfully speaking, clinical trials are expensive ventures, for instance, Pfzier, an American pharmaceutical and biotechnology corporation based in the US, that manufactures and produces the Pfzier Covid-19 vaccine that was found by the Food and Drug Administration - a body responsible for protecting public health by ensuring the safety, efficacy and security of human and veterinary drugs, to be 95 per cent efficacious in the treatment and management of Covid-19, is reported to have received about USD 1.9 billion (about Shs6.84 trillion) for vaccine development, whereas Moderna, whose vaccine efficiacy rate stands at 94.1per cent, is reported to have received $483 million (about Shs1.739 trillion) from the U.S government for vaccine development.
As such, without the much needed funding to fund such expensive ventures, scientists in Uganda simply look out for the active ingredients, mix them and create efficacious drugs and this is why we have seen the birth of Covidex and Covilyce.
Therefore, NDA should create a swift mechanism that enables it to efficiently and effectively examine herbal medicines before they are made available to the general public. The FDA in USA has a strict legal regime and it has been swift in approving covid-19 vaccines such as Pfzier and Moderna, and am certain that the NDA can equally do the same if not, better when it comes to approving herbal medicines.
Paul Wasswa, [email protected]