NDA warns of counterfeit malaria drugs on market
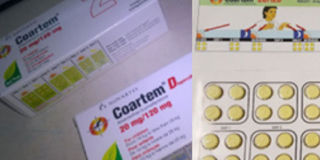
The genuine Green Leaf Coartem in a blister in a box pack. Photo by Emmanuel Ainebyoona
KAMPALA- The National Drug Authority has cautioned health workers against dispensing and selling counterfeit anti-malarial drugs that have flooded the market.
According to Mr Fredrick Ssekyana, the NDA spokesperson, counterfeit Coartem with the Green Leaf AMFm wallet packs have been found on the market with the following pack details; Batch number of NOF 2153, date of manufacture of June 2015 and expiry date of July 2018.
Mr Ssekyana said: “NDA Quality Control Laboratory tested the Wallet Pack Counterfeit Coartem and results indicated no active ingredients of Artemether or Lumefantrine.”
He added that the wallet packs of 24 tablets bear a shelf life of three years, which is not representative of the genuine Coartem whose shelf life is two years.
“We therefore advise all health professionals not to stock, buy or dispense the above packaging of counterfeit Coartem as it poses grave danger to the public and should not be on sale in any outlet,” Mr Ssekyana warned.
A statement released by NDA yesterday indicated that Novartis Pharmaceuticals, the manufacturer of the genuine Coartem, through the local technical representative Surgipharm Uganda Ltd stopped the supply of the Green Leaf AMFm Wallet pack Coartem to the private sector in 2014.
“Effectively no wallet packs whatsoever, should be on sale in the private sector,” the NDA statement added. Mr Ssekyana said investigations are underway to establish the source of the counterfeit drugs.
“It is a criminal offence to deal in counterfeit products as they greatly endanger the lives of the population. Should you come across any of the aforementioned packs or any other suspicious packs, urgently report to NDA,” he added.
He said the genuine Coartem of the type are only available in Public health facilities, not private facilities.




