Covid drugs scientists struggle to meet terms for clinical trials
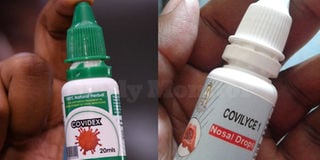
Covidex (R), the herbal medicine that was approved by the National Drug Authority as a supportive treatment for Covid-19 and Covilyce1, a herbal medicine that is being produced by Gulu University. PHOTO/FILE
What you need to know:
- Developers of Covidex and Covilyce-1 had said in July that they were set to subject their products to clinical trials.
The National Drug Authority (NDA) has blamed the delayed start of clinical trials for locally made Covid-19 medicines on the developers, saying they have not met the set requirements.
This comes three months after President Museveni directed that locally developed Covid drugs should meet globally prescribed standards for drug development, including clinical trials.
He said this was to protect Ugandan products from being “labelled inferior and sabotaged by the international pharmaceutical players.”
Mr Abiaz Rwamwiri, the NDA spokesperson, on Friday said no developer of herbal medicines has met the laid down standards to be allowed to start clinical trials. “The lead investigator of Covidex, submitted an initial proposal, and the committee [for clinical trials approval] sat and reviewed it on September 20. The report was sent back on
September 23 to the lead investigator with feedback, demanding some clarifications and different answers and NDA has not seen any response from the lead investigator to date,” he said.
He didn’t elaborate, however, sources at NDA said the application for NDA approval costs $2,500 (about Shs9m).
“If he had applied officially to the research ethics committee, we could have seen the application,” another source said.
The developers of Covidex and Covilyce-1, which are being used as supportive treatment for Covid-19, had said in July that they were set to subject their products to clinical trials.
There are at least four well-known products being used as supportive treatment for Covid-19 and some are yet to be approved by NDA.
Prof Patrick Ogwang, the developer of Covidex, when asked about the progress on clinical trials, said: “We are still pursuing clearance of the study by NDA and Uganda National Council for Science and Technology.”
Dr Alice Lamwaka, the lead developer of Covilyce-1, referred this reporter to the Ministry of Science and Technology when asked when the trials will start.
Mr Rwamwiri said Covilyce-1 has not even met the first criteria for drug approval for use as a supportive treatment.
He said the medicine will need to undergo the steps, but that Covidex has already been given the initial approval, according to NDA.
“The National Drugs Authority with other regulators of clinical trials formed a joint review committee, which is under the secretariat of the Uganda National Council for Science and Technology, the Uganda National Research Organisation, Research Ethics Committee, and an expert on the subject that is going to be under study,” he said.
Mr Rwamwiri said the joint review committee will avoid the lead investigator appearing before different regulators many times so as to expedite clinical trials.
“For us to approve [a clinical trial], that official application after the joint committee has approved and the research ethics committee has cleared you, then NDA looks into it. But NDA has not yet received an official application,” Mr Rwamwiri said.
Dr Monica Musenero, the minister for Science, Technology and Innovations, couldn’t be reached for comments on whether they had released the funds that the President had promised the drug developers to support the trials.
Prof Ogwang and Dr Lamwaka had said earlier that the funds had not yet been released.
But Prof Ogwang said his company was availing part of the funds.




