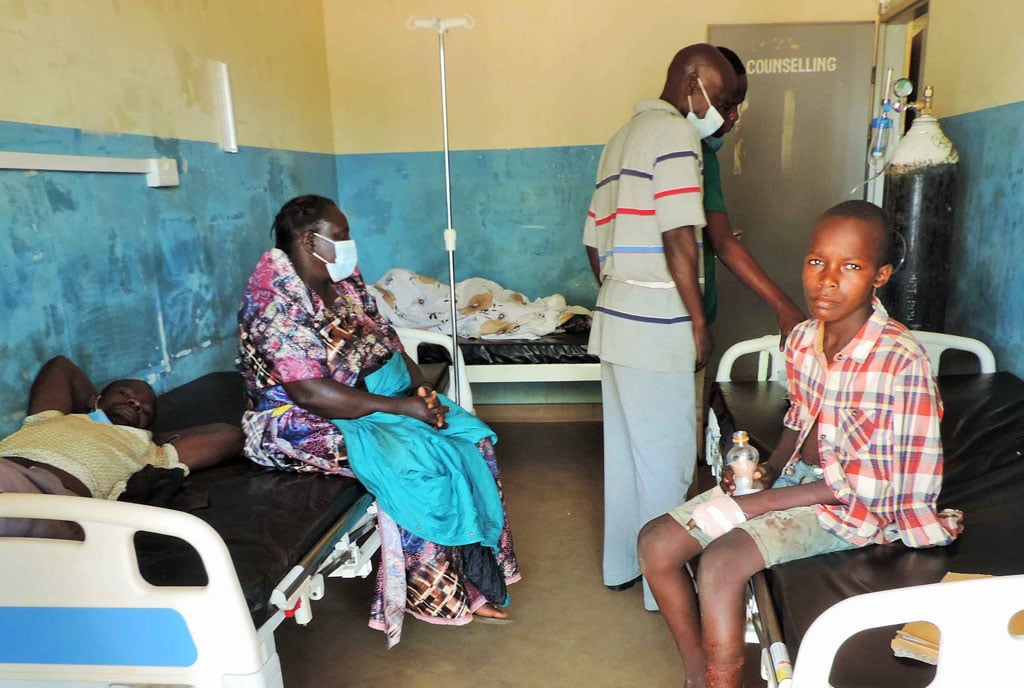
Antibiotic resistance can be prevented

Before taking any medication, one must get proper assessment from a doctor. PHOTO/www.istockphoto.com
What you need to know:
- To effectively reduce AMR, all sectors must use antimicrobials prudently and appropriately, take preventive measures to decrease the incidence of infections and follow good practices in disposal of antimicrobial contaminated waste.
Celebrated from November 18-24 every year, the World AMR Awareness Week (WAAW) is a global campaign to raise awareness and understanding of Antimicrobial Resistance and promote best practices among health stakeholders to reduce the emergence and spread of drug-resistant infections.
What is AMR?
AMR occurs when bacteria, viruses, fungi and parasites no longer respond to therapeutic substances used to prevent or treat infections (antimicrobial agents). As a result, the treatments become ineffective and infections become expensive and difficult to treat, increasing the risk of disease spread, severe illness and sometimes, death.
For instance, if one develops multi-drug resistant tuberculosis, they are switched from first line drugs, whose dosage is usually complete in six months to second line drugs that are more expensive and take about 18 to 20 months for one to complete treatment.
According to Dr Sabrina Kitaka, a paediatric infectious disease specialist, AMR is a threat to humans, animals, plants and the environment.
“We are increasingly seeing patients with resistant infections, especially among children. These infections are becoming more difficult to treat yet there are no new antibiotics being developed. Discovering new antibiotics and bringing them to market is a formidable challenge. It can take 10 to 15 years and it is very costly,” she says.
Situation
The Uganda National Academy of Sciences (UNAS) undertook a situational analysis on antimicrobial resistance in Uganda. The report found increasing trends in antimicrobial resistance.
According to the Ministry of Health Annual Health Sector Performance Report, microbial infections, including pneumonia, tuberculosis and sepsis accounted for 18.4 percent of hospital-based mortality. Of those, pneumonia was the biggest contributor at 9.7 percent.
Additionally, microbial infections were responsible for 37 percent of all hospital admissions. Resistance to the most commonly-used antimicrobials (for example penicillins, tetracyclines, cotrimoxazole) was in some cases above 80 percent. Of particular concern was the report of the high prevalence of multi-drug resistant bacteria such as methicillin-resistant staphylococcus aureus (MRSA) and extended-spectrum beta-lactamase (ESBL)-producers.
The African antibiotic treatment guidelines for common bacterial infections and syndromes set guidelines to provide healthcare workers with expert recommendations for antimicrobial selection, dosage and duration of treatment for common bacterial infections and syndromes among paediatric and adult patient populations in Africa and to promote the appropriate use of antimicrobials to mitigate the emergence and spread of AMR pathogens.
Antibiotic consensus principles
Reducing AMR requires a multi-sectoral approach and in 2010, paediatricians serving at the department of paediatrics at Makerere University and Mulago National Referral Hospital started the Antibiotic Consensus Society of Uganda to provide stewardship for correct antibiotic use, prevent antibiotic resistance in communities and improve patient outcomes.
Whereas it is widely accepted that inappropriate antibiotic usage contributes to AMR, it is difficult to change prescribing behaviour. The society helps to identify principles underlying appropriate antibiotic prescribing and guideline formulation to reduce morbidity from community-acquired respiratory tract infections, limit therapeutic failure and most importantly, curb AMR emergence.
According to Dr Kitaka, the first and most important guiding principle is that antibiotics should only be used to treat bacterial infections.
She adds that high levels of prescribing for non-bacterial infections such as the common cold, particularly in primary care, are a common practice. The main reason noted is the pressure from patients and parents along with constraints on physician time.
There is also a need to optimise diagnosis and severity assessment and a practical criterion is needed to identify bacterial infections that require antibiotic therapy. Lack of availability of cost-effective antimicrobial resistance.
She recommends carrying out a blood, urine and tissue culture from a patient before prescribing antibiotics. The culture will help the doctor know what antibiotics will effectively treat a particular infection.
Antibiotic therapy should reduce maximally or eradicate the bacterial load since evidence confirmed that bacterial eradication should be the goal of antibiotic therapy. Therefore, antibiotics should be given with the aim of maximising bacterial eradication.
Health workers should recognise and act on local resistance prevalence. Increasing high-level resistance in respiratory tract infection pathogens must result in increased therapeutic failures but the extent of failure in community-acquired respiratory tract infections in relation to resistance prevalence is unknown. Prescribing should, therefore, be appropriate in the type and context of local resistance prevalence.
Effective dosing
“As health workers, we do not just give antibiotics. Prescribing should be based on pharmacodynamic principles that can predict efficacy (bacterial eradication) and thus, limit the emergence of resistance,” Dr Kitaka says.
The principles also include integrating local resistance, efficacy and cost-effectiveness. Pharmaco-economic analysis had confirmed that more-effective antibiotics can reduce overall management costs, particularly concerning consequential morbidity and hospital admission.
Health workers should also be professional enough and carry out a proper diagnosis, correctly interpret the results and explain them to the patient in a way they understand it best. It is important that one understands why you are giving them a particular drug.
Role of patients
Whereas the antibiotic consensus principles concern the efforts of health workers, patients also have a role to play in preventing and reducing the burden of drug resistance according to Dr Amos Okoth Onegi, a clinical pharmacist at Mulago National Referral Hospital.
Drug resistance is a public health challenge. Tuberculosis is on the rise and it is very easy for one to develop resistance to the drugs that treat the disease. MDR TB is a local and global challenge which is expensive to treat and takes a lot of time for one to complete this medication.
“Patients have a role to play,” Dr Okoth says. “There are simple preventive mechanisms that are not being embraced. Wearing masks, handwashing and keeping in well aerated spaces would reduce one’s risk of developing TB but also, when one tests positive for the disease, they should complete their medication as prescribed by the doctor.”
According to Dr Charity Mubeezi, a supply chain consultant and global health specialist, many people will take medications based on advice from friends. However, she says, one must get proper assessment from a doctor before they start taking any medication.
Caution
Take antibiotics exactly as prescribed if you need them. If your doctor decides an antibiotic is the best treatment when you are sick:
•Take them exactly as your doctor tells you.
•Do not share your antibiotics with others.
•Do not save them for later. Talk to your pharmacist about safely discarding of leftover medicines.
•Do not take antibiotics prescribed for someone else. This may delay the best treatment for you, make you more sick, or cause side effects.
Talk to your doctor if you have any questions about your antibiotics.