NDA links illegal “Chinese” contraceptive pill to cancer, infertility
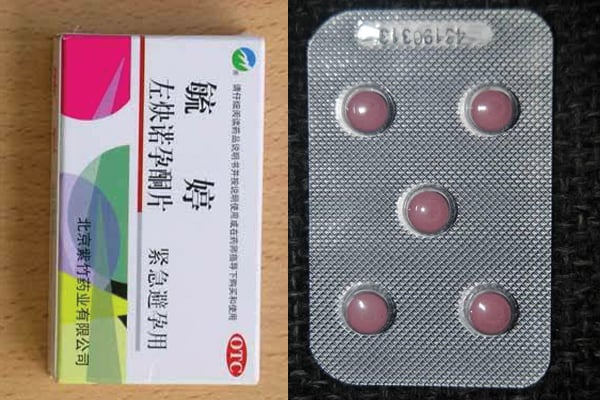
The Chinese Pill tablet packaging, labelling and patient information leaflet are in the Chinese language except for the claimed ingredients –Levonorgestrel and Quinestrol, according to NDA. PHOTOS/ COURTESY
The National Drug Authority (NDA) has warned drug outlets across the country to stop stocking and sale of the unauthorised “Chinese” contraceptive pill with immediate effect.
The drug regulator said Friday that the unauthorised pill, which predisposes consumers to cancer and infertility risks, is being sold on the black market.
“The Chinese Pill tablet packaging, labelling and patient information leaflet are in the Chinese language except for the claimed ingredients –Levonorgestrel and Quinestrol,” reads the notice undersigned by Dr David Nahamya, the Secretary to the Authority.
READ:
Levonorgestrel and Quinestrol are hormones in birth control pills.
“The pill was found to contain a high dose of the hormone above recommended dosage and the risks associated with the use of this product include among others; prolonged bleeding, irregular menstrual periods,” Dr Nahamya said in the notice.
He added that the other risks include “palpations, possibility of developing blood clots and heart diseases, abnormally thickened endometrium, predisposing factor for endometrial cancer and infertility.”
According to NDA, when consumed, the hormones stay in the body for a long time and the adverse effects of the pill further manifest in babies that are born by mothers.
“The adverse effects include secondary sexual characteristics like premature puberty. NDA strongly warns all drug outlets or persons with immediate effects to stop stocking and sale of the Chinese pill,” Dr Nahamya said.
He added: “NDA post-market surveillance and enforcement units are on full alert to undertake regulatory actions against this illegality. NDA advises the public to stop the consumption of this product whose safety and quality cannot be guaranteed.”
Quality family planning services are provided by qualified health professionals in registered health facilities.
“The public is expected to remain vigilant and report any suspected substandard and falsified medical products to NDA,” the regulator added.
[email protected]




